Industry-First Electric Vaccine Vehicle Launches to Expand Access to COVID-19 Vaccination and Testing
In response to the ongoing challenges facing vaccine distributors, segment leaders Element, Club Car, AYRO and Gallery Carts have teamed up to develop a compact, zero-emissions vehicle offering that helps make vaccines more accessible
AYRO, Inc. a designer and manufacturer of light-duty, short-haul, and last-mile delivery electric vehicles (EVs), today announced the launch of the Electric Vaccine Vehicle (EVV) www.vaccineEV.com , which is designed specifically to mobilize a flexible, safe and efficient means of delivering vaccines and testing to millions of people in 2021 and beyond.
Element Fleet Management Corp. (“Element”), the world’s largest pure-play automotive fleet manager will offer sales, financing solutions and vehicle management services throughout the vehicle lifecycle to EVV fleet operators. Club Car, one of the most respected names in the golf and utility vehicle industry, will provide sales and service support for the vehicles to Element and its Clients. Each company will leverage its national footprint and expertise to facilitate the deployment of EVVs and meet urgent demand.
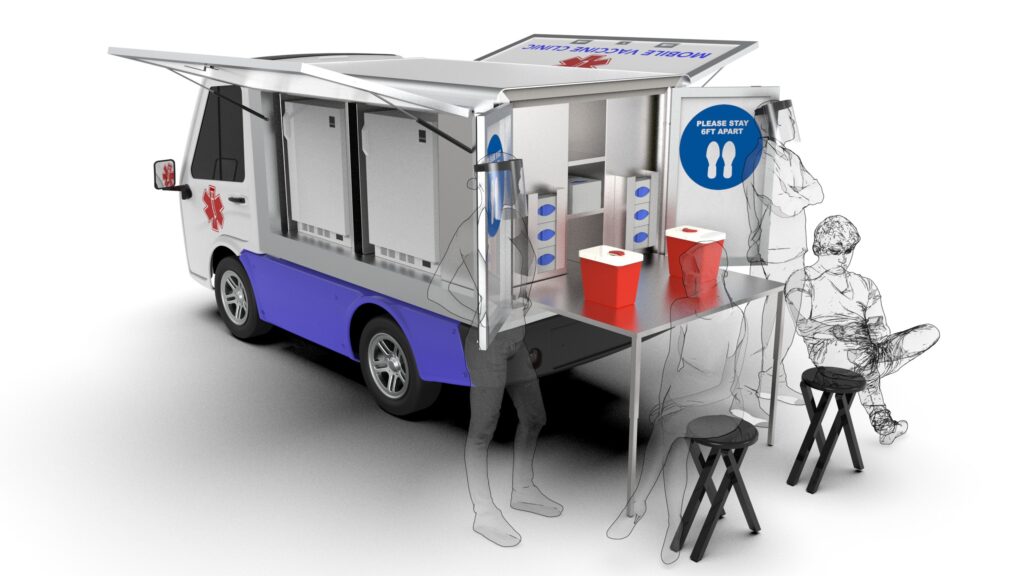
The EVV is a self-contained, all-electric transportation solution that has been optimized to store, transport, and deliver testing and vaccines to a large population across a wide variety of environments and locations. The zero-emissions vehicle features medical-grade equipment and a made-to-order design by AYRO and mobile cart’s leader Gallery, expanding on the companies’ existing food service vehicle models. When partnered with community healthcare systems, it makes safe and rapid COVID-19 testing and vaccine distribution possible by reaching patients in need and managing demand at aggregation centers. It can traverse tight areas and help reduce wait times at central distribution locations by meeting patients at their place in line. When configured as a street-legal vehicle it can serve populations unable to get to vaccination sites because they don’t have access to transportation —including millions of high-risk older adults and low-income households.
Additional EVV benefits include:
- Medical-Grade Equipment — Each EVV is outfitted with an ultra-low temperature freezer and refrigeration units with Bluetooth-enabled data loggers and temperature monitoring devices to virtually track and log live inside readings and pharmaceutical conditions, which follows the Centers for Disease Control & Prevention (CDC) guidelines to safely store and distribute vaccines.1 There is also on-board medical-grade storage, mobile onboard sinks, and other features designed to meet the needs of mobile healthcare providers and patients.2
- Zero-Emissions, Zero Exhaust — The 100% electric vehicle can be moved into and operated indoors to allow medical providers to treat patients virtually anywhere without greenhouse gas (GHG) emissions or fumes.
- No Specialized EV Charging Infrastructure Required — The EVV has a 50-mile driving range plus approximately 6-8 hours of equipment operation on a single charge (depending on EVV configuration and driving conditions), or can provide all-day operations if plugged in. Each vehicle can be charged and/or operated using a standard 110V/20amp outlet, so no EV charging infrastructure is required.
- Compact Size — The vehicle enables more patient access, even within space-constrained locations. Each vehicle as a vaccine station takes up less than 100 square feet, allowing for easy group deployments. Vaccine administration can also happen while the patient is still inside his or her own vehicle or directly outside of the EVV for a rapid, drive-through experience.
- Street Legal EVV Configuration— Select EVV configurations can be registered and licensed to operate at 25 MPH on city streets with posted speeds up to 35 MPH. The low-speed EV can also maneuver through car-free zones bringing essential services directly to patients that need them. Check your local regulations and ordinances as to whether the EVV may be operated legally on public streets, and if so, under what conditions.
The EVV can also be adapted for future needs—disaster relief, annual flu shot distribution, medical testing, food services and more — with clearly identifiable vehicle branding customized to convey each use case. The vehicle can also be leased rather than purchased reducing the impact to capital budgets, while also providing flexibility in the lifecycle. The EVV Roadshow is taking place across major U.S. cities in March and April www.vaccineEV.com for local governments and organizations to experience the vehicle in-person.
“Element is pleased to bring our broad network, scalable operating platform and industry-leading strategic consulting services to support our existing and prospective clients interested in the EVV ”, said David Madrigal, Executive Vice President and Chief Commercial Officer. “We look forward to being part of the Club Car, AYRO and Gallery teams helping to bring an innovative solution to vaccine distribution.”
“This initiative allows us to tap our network of industry leading dealers to ensure the Electric Vaccine Vehicle is ready to deliver essential vaccines quickly,” said Jeff Tyminski, Vice President of Product Management and Marketing at Club Car. “Our extensive local customer support and service coverage allows us to provide the EVV solution to areas in need immediately.”
“COVID-19 testing and vaccine distribution has become a serious logistical challenge and our purpose-built EVs offer a potential solution,” said AYRO CEO Rod Keller. “Throughout the pandemic, we’ve been working with our partners to design customizable EVs for food delivery on college campuses in lieu of crowded dining halls. We quickly realized that experience designing EVs with hot and cold storage and hygiene precautions translated well to mobile vaccination vehicle design. The Electric Vaccine Vehicle can go virtually anywhere — within buildings or throughout cities and parking lots — potentially bringing millions of vaccines to patients.”
“The Electric Vaccine Vehicle is our most important and impactful customizable solution to date. The EVV overcomes a wide range of physical and logistical barriers, and will enable making vaccine distribution, testing resources and other medical services more accessible to as many people as possible,” said Dan Gallery V at Gallery Carts. “Rather than bringing the people to the medical services, we’re now bringing the medical services to the people.”
Learn more about the solution and inquire about orders at www.vaccineEV.com.
1 The CDC requires a data logger or temperature monitoring devise to consistently record live temperature readings from the inside of a refrigerator or freezer when storing all types of vaccines. Constant monitoring of vaccines at recommended temperatures is essential for maintaining the efficacy of a vaccine. Failing to do so can lead to wasted vaccines or ineffective vaccines being administered to patients. Please follow the CDC requirements for Vaccine Storage & the COVID-19 Vaccination Program found here.
2 All medical grade refrigerator and freezer units tested and certified to maintain temperatures within refrigerated or frozen ranges required by the CDC.
Category: Cab, Trailer & Body, Equipment, Featured, News, Products, Safety, Vehicles